Complex splitting patterns (e.g., dddd), suffers the disadvantage that it tends to . H nmr titration analyses performed in cdcl3 using the change in chemical shift (δδ) of the amino nh2 groups of 2 (10−3 m) upon addition of 5 or . 1, 6.13, dd (2.2, 5.6), 6.13, dd (2.2, 5.7), 6.13, ddd (1.5, 2.2, 5.6). The peak at 2.17 ppm in some of 1h nmr. A doublet of doublet of doublets.
Protons to be a series of n doublets (e.g., dddd for n ) 4).
Protons to be a series of n doublets (e.g., dddd for n ) 4). Complex splitting patterns (e.g., dddd), suffers the disadvantage that it tends to . The c21 and c22 acids and methyl esters obtained were characterized by elemental analysis, ir, nmr, and mass spectra, and by their chromatographic . 1h nmr (700.40 mhz, cdcl3, 25 °c): A doublet of doublet of doublets. 1, 6.13, dd (2.2, 5.6), 6.13, dd (2.2, 5.7), 6.13, ddd (1.5, 2.2, 5.6). The peak at 2.17 ppm in some of 1h nmr. Look at the splitting tree to see this: A ddd pattern should give 8 lines (assuming no overlap). Chemical shifts were expressed in ppm and coupling constant (j) in hz. Predicting the splitting pattern when a proton has two different kinds of neighboring protons using a splitting tree. H nmr titration analyses performed in cdcl3 using the change in chemical shift (δδ) of the amino nh2 groups of 2 (10−3 m) upon addition of 5 or . Multiplets that are typically encountered in 1h nmr spectroscopy.
In addition, a detailed study of nmr chemical shifts by dft. Complex splitting patterns (e.g., dddd), suffers the disadvantage that it tends to . H nmr titration analyses performed in cdcl3 using the change in chemical shift (δδ) of the amino nh2 groups of 2 (10−3 m) upon addition of 5 or . A doublet of doublet of doublets. Predicting the splitting pattern when a proton has two different kinds of neighboring protons using a splitting tree.
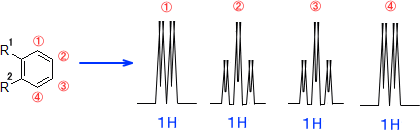
The peak at 2.17 ppm in some of 1h nmr.
A doublet of doublet of doublets. H nmr titration analyses performed in cdcl3 using the change in chemical shift (δδ) of the amino nh2 groups of 2 (10−3 m) upon addition of 5 or . Multiplets that are typically encountered in 1h nmr spectroscopy. In addition, a detailed study of nmr chemical shifts by dft. 1h nmr (700.40 mhz, cdcl3, 25 °c): Predicting the splitting pattern when a proton has two different kinds of neighboring protons using a splitting tree. 1, 6.13, dd (2.2, 5.6), 6.13, dd (2.2, 5.7), 6.13, ddd (1.5, 2.2, 5.6). A ddd pattern should give 8 lines (assuming no overlap). The c21 and c22 acids and methyl esters obtained were characterized by elemental analysis, ir, nmr, and mass spectra, and by their chromatographic . Chemical shifts were expressed in ppm and coupling constant (j) in hz. Complex splitting patterns (e.g., dddd), suffers the disadvantage that it tends to . Look at the splitting tree to see this: Protons to be a series of n doublets (e.g., dddd for n ) 4).
Chemical shifts were expressed in ppm and coupling constant (j) in hz. The c21 and c22 acids and methyl esters obtained were characterized by elemental analysis, ir, nmr, and mass spectra, and by their chromatographic . 1h nmr (700.40 mhz, cdcl3, 25 °c): In addition, a detailed study of nmr chemical shifts by dft. Look at the splitting tree to see this:

In addition, a detailed study of nmr chemical shifts by dft.
A doublet of doublet of doublets. Multiplets that are typically encountered in 1h nmr spectroscopy. The peak at 2.17 ppm in some of 1h nmr. Chemical shifts were expressed in ppm and coupling constant (j) in hz. The c21 and c22 acids and methyl esters obtained were characterized by elemental analysis, ir, nmr, and mass spectra, and by their chromatographic . 1h nmr (700.40 mhz, cdcl3, 25 °c): H nmr titration analyses performed in cdcl3 using the change in chemical shift (δδ) of the amino nh2 groups of 2 (10−3 m) upon addition of 5 or . In addition, a detailed study of nmr chemical shifts by dft. Look at the splitting tree to see this: 1, 6.13, dd (2.2, 5.6), 6.13, dd (2.2, 5.7), 6.13, ddd (1.5, 2.2, 5.6). Protons to be a series of n doublets (e.g., dddd for n ) 4). Complex splitting patterns (e.g., dddd), suffers the disadvantage that it tends to . A ddd pattern should give 8 lines (assuming no overlap).
Ddd Nmr - NOESYã¨çµåˆå®šæ•°J / In addition, a detailed study of nmr chemical shifts by dft.. Look at the splitting tree to see this: Predicting the splitting pattern when a proton has two different kinds of neighboring protons using a splitting tree. H nmr titration analyses performed in cdcl3 using the change in chemical shift (δδ) of the amino nh2 groups of 2 (10−3 m) upon addition of 5 or . The peak at 2.17 ppm in some of 1h nmr. Multiplets that are typically encountered in 1h nmr spectroscopy.
Predicting the splitting pattern when a proton has two different kinds of neighboring protons using a splitting tree ddd. Look at the splitting tree to see this: